Contact:
Gina Giorgio
Director of Strategy and Development
Students for Sensible Drug Policy
gina@ssdp.org
DEA SILENCES SCIENTISTS; STUDENT GROUP PUSHES BACK AGAIN
Since 2022, Students for Sensible Drug Policy (SSDP) Has Led the Fight to Prevent the DEA From Scheduling DOI and DOC, Which Would Create Serious Impediments to Potentially Life-Saving Research
[Washington D.C., October 3, 2024] —
Students for Sensible Drug Policy (SSDP)—a leading nonprofit representing over a dozen graduate students, post-docs, staff scientists, and professors—has filed a motion in the Drug Enforcement Agency (DEA) administrative Court today pushing back on the DEA’s latest efforts to silence scientists.
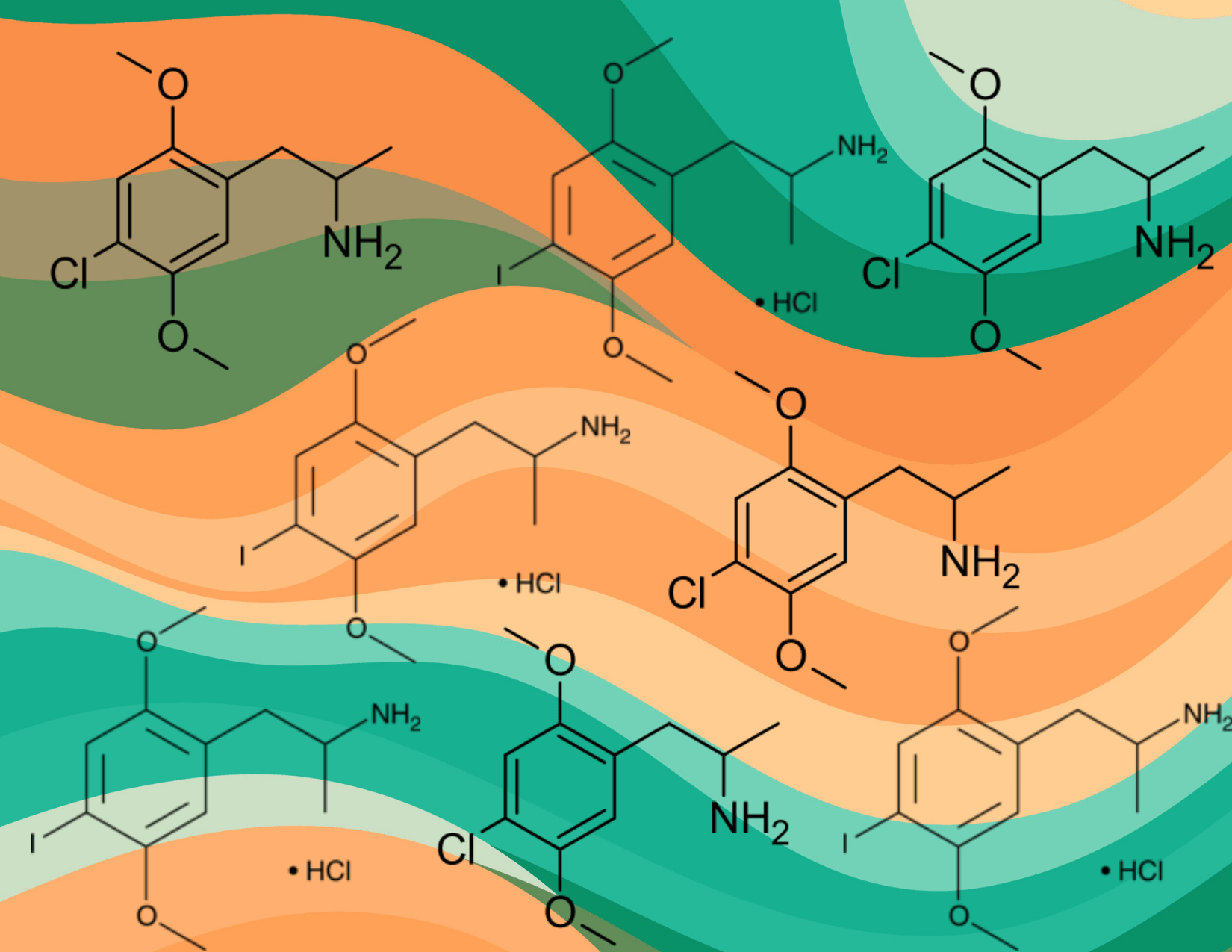
The legal filing is in response to a motion filed by the DEA last week which would silence expert witnesses and block crucial scientific evidence from coming to light in an upcoming public hearing on the proposed scheduling of 2,5-Dimethoxy-4-iodoamphetamine (DOI) and 2,5-Dimethoxy-4-chloroamphetamine (DOC), two research chemicals that have been used safely in labs since the early 1970s.
“What is the DEA so scared of that these leading scientists must be muzzled?” says Robert Rush, a Denver-based attorney who is working hand-in-hand with SSDP in a joint effort to keep research using DOI and DOC legal.
“By trying to silence the world’s leading experts in neuroscience and pharmacology, the DEA is attempting to prevent the tribunal—and the public—from understanding the true potential of these substances,” says Rush. “We vigorously oppose the DEA’s efforts to stop scientific research and call on the tribunal to reject the Government’s motion and ensure that all relevant evidence is heard.”
Research Harm
The DEA’s motion to prevent testimony from SSDP’s Science Policy Committee and other prominent researchers, raises serious concerns about transparency and the integrity of the decision-making process. Excluding these scientific experts not only undermines the fairness of the hearing but could prevent vital evidence from being heard—evidence showing how the proposed scheduling would stifle research into the therapeutic potential of DOI and DOC.
“The Government argues that research harm evidence is not relevant,” said the DEA in a motion filed on September 26th to Administrative Law Judge Paul E. Soeffing. “Permitting the qualification of scientific experts merely to discuss ‘research harm’… would result in a waste of judicial time and resources.”
The DEA’s assertion has raised alarm among scientists and advocates, who argue that excluding key evidence related to research harm ignores the broader consequences of restricting access to DOI and DOC.
“We have assembled a world-class international group of behavioral and molecular pharmacologists, chemists, neuroscientists, and a former Government Advisor on psychoactive drugs,” says Elijah Ullman, a Ph.D. Candidate in Molecular and Systems Pharmacology at Emory University and chair of SSDP’s Science Policy Committee, who has led SSDP’s ongoing efforts to keep DOI and DOC from being added to the Controlled Substances Act since 2022.
“If the DEA believes that both DOI and DOC should be placed in Schedule 1, they should have no issue allowing our witnesses to testify in full—after all, don’t they allege they have sufficient data to prove DOI and DOC are addictive and a harm to the public?” says Ullman. “If the DEA believed their own rhetoric and description of the data, they would allow our proposed witnesses to testify in full. Otherwise, it appears to me that they are worried their dataset is lacking the scientific rigor to support their claims.”
The Importance of DOI and DOC in Research
The outcome of this hearing could have long-lasting implications for scientific freedom and public health. DOI and DOC are essential research chemicals in pre-clinical psychiatry and neurobiology whose status as unscheduled compounds has made them de facto tools for researchers studying serotonin receptors.
DOI in particular has been a cornerstone in neuroscience research due to its high selectivity for the 5-HT2A serotonin receptor, a critical component in understanding and potentially enhancing the therapeutic effects of psychedelics.
DOI’s use in the lab ushered in a new era of psychiatric drug discovery since it was used to map the localization of an important serotonin receptor in the brain critical in learning, memory and psychiatric disease. Over 80% of the antidepressant drugs on the market affect the serotonin system.
Over the past two decades, DOI has been cited in more than 900 research articles, contributing significantly to our understanding of complex neural mechanisms and offering potential pathways for breakthrough treatments. Recent studies utilizing DOI have shown encouraging results in managing pain and reducing opioid cravings, offering a beacon of hope in the ongoing opioid crisis.
“At a time when we face an unprecedented mental health crisis, it is unconscionable for the DEA to claim that harm to psychedelic research is irrelevant,” says Rush. “I look forward to the DEA being called to explain themselves in blocking critical research that so many Americans struggling with serious issues may benefit from.”
Limiting the scope of expert testimony would undermine the ability of researchers to illustrate the profound impact that DOI and DOC could have on scientific discovery and public health. Many believe that this move is an attempt to sidestep critical conversations about how scheduling decisions affect the future of groundbreaking medical research.
“I have spent an entire Ph.D. in Neuroscience studying DOI. The main effect of placing DOI and DOC in Schedule I will be taking a step backwards in neuroscience research,” says Tanner Anderson, a Ph.D. Candidate in Neuroscience at the University of Kentucky College of Medicine, a member of SSDP’s Science Policy Committee, and one of the key witnesses that the DEA is attempting to silence.
Anderson has published data observing the effects of DOI on neuronal signaling and received an F31 training grant from NIDA to study the neuronal and behavioral effects of DOI in models of substance use disorder.
“As a scientist who would be impacted by this scheduling, why should I be excluded from the upcoming public hearing?,” asks Anderson. “It makes no sense to exclude the only people at the hearing that study these drugs.”
DEA Attempts to Silence the Science
The ten day long public hearing, scheduled to occur November 12-25 at the DEA Headquarters in Arlington, Virginia, was previously expected to include a detailed review of both the potential risks and benefits of DOI and DOC.
The DEA’s move to exclude expert testimony undermines the integrity of the scientific process, particularly in a case of such broad-reaching implications for public health.
“The DEA, for too long, has had a rubber stamp in their decision-making processes. They have not had to defend their actions,” says Rush. “I am looking forward to having the DEA defend its decision to criminalize an important research tool that it admits is not being diverted for illicit use. It is time they answered to the American public.”
SSDP and other groups argue that the research being done on these substances is critical not only for understanding psychedelics, but for advancing neuroscience and mental health treatment overall.
“Our goal is to ensure that the most qualified experts are able to present evidence on the broader implications of this decision,” said SSDP Director of Strategy and Development Gina Giorgio. “We question the fairness and credibility of a DEA-led trial that excludes the world’s foremost experts from participating in the discussion.”
Students Lead the Charge
The fight to preserve access to DOI and DOC has been spearheaded by young researchers, many of whom are currently engaged in Ph.D. work and the majority of whom are members of SSDP’s Science Policy Committee.
“In SSDP, I found no shortage of mentors to guide me through the political process of getting involved, active, and helping to pass legislation,” says Ullman. “The organization taught me how a group of people can come together to change the world.”
Founded in 1998, SSDP has mobilized and empowered thousands of young people of all political and ideological orientations to participate in the political process and advocate for a more sensible approach to drug laws.
“SSDP has always empowered young people driven by a passion for science and justice to be the catalyst for change,” says SSDP Executive Director Kat Murti, who first joined SSDP as an undergraduate student at the University of California at Berkeley in 2009. “The researchers that the DEA is attempting to muzzle have shown that students, when given the right tools and support, can drive significant policy shifts and protect the integrity of scientific research. They are not just participants; they are leaders in the fight to defend the truth and expand our understanding of human health.”
Ullman, who joined SSDP as a Sophomore in high school in 2012 and has been fighting to preserve scientific access to DOI and DOC since 2022 as Chair of SSDP’s Science Policy Committee, will defend his Ph.D. thesis soon after the hearing.
“With the upcoming hearing, we have the once-in-a-generation opportunity to stand up for the scientific community and reasonably communicate data,” says Ullman. “I am excited to be a scientific steward in protecting researcher’s abilities to understand human health.”
With chapters on campuses and in communities across the country, Students for Sensible Drug Policy (SSDP) is the largest national youth-led network dedicated to ending the War on Drugs. Our national staff, Board of Directors, chapters, and alumni work together to replace the disastrous War on Drugs with policies rooted in evidence, compassion, and human rights, at a grassroots level.
###
For more information, please visit: https://ssdp.org/dont-block-psychedelic-research/